It seems an age has passed since Saturday when the second Forum on Education was held at St Albans School. Many ideas have been whizzing about my head since listening to the keynotes and popping in and out of the seminars (of course one of the great draw backs to organising a conference is not being able to attend sessions in full). The theme of Learning Relationships seemed to knit together the different topics on offer, with unusual perspectives giving a reminder of what is actually important for learners and teachers.
It seems an age has passed since Saturday when the second Forum on Education was held at St Albans School. Many ideas have been whizzing about my head since listening to the keynotes and popping in and out of the seminars (of course one of the great draw backs to organising a conference is not being able to attend sessions in full). The theme of Learning Relationships seemed to knit together the different topics on offer, with unusual perspectives giving a reminder of what is actually important for learners and teachers.
I would just like to take this opportunity to thank everyone present, delegates and speakers alike, for making the day so enjoyable to host. The conversations that flowed showed just how important it is to take time to reflect on and discuss what we do, emphasising keynote speaker Mike Grenier’s focus on slow education. The fact that all of the speakers were teachers, actually at the coal face of education, brought an honesty that can be hard to mimic. Certainly my one big ‘takeaway’ from the day is that education is in good hands. It is an absolute pleasure to have helped set up the right  environment to enable us to come together. With huge thanks to the cleaners, porters, catering team and John in reprographics who all enabled the conference to go ahead. And I would single out Rob Hagon and Network Support for their hard work in allowing the IT to be invisible throughout the day.
environment to enable us to come together. With huge thanks to the cleaners, porters, catering team and John in reprographics who all enabled the conference to go ahead. And I would single out Rob Hagon and Network Support for their hard work in allowing the IT to be invisible throughout the day.
The St Albans School Forum on Education 2017 has set a high bar and following the success of the day, we will be looking to host our third conference at a similar point in the Summer Term 2018. Once again tickets will be in limited supply to ensure we retain the intimate and small-scale atmosphere that makes SASFE so special. Watch out for updates in September and January!

Are you looking for speakers at your next conference or teachmeet?
I would whole-heartedly recommend all of the following, arranged in alphabetical order.
- Dr Jill Berry – closing the conference with her keynote on how leadership can be used to maintain and improve working relationships within a school.
- Elizabeth Carr – explored the role of the subject in learning relationships and how to model history as a discipline.
- Dawn Cox – discussed how whole school initiatives need to be fully focused on learning and linked to research in her seminar titled ‘Where’s the learning?’
- Andy Ford and Tom Hockedy – a session that presented the progress of an Action Research Project at Berkhamsted School trying to answer the question ‘If the learning environment is more advanced, will it create more powerful learners?’
- Mike Grenier – opening the conference with a keynote on how Slow Education can help develop learning relationships in a school.
- Dr Greg Hacksley – joined by St Albans School Upper Sixth pupils Dan, Darcey, Josh and Paddy this session took a pupil’s-eye view of education to answer the question ‘How do I learn?’
- Matt Pinkett – used a mixture of anecdotal evidence, personal experience and research to argue that there is a masculinity crisis in our schools with suggestions of what schools can do to address it head on.
- David Rogers – discussed the concept of grit, detailing a long term project that aims to develop academic resilience to give pupils the qualitites and qualifications needed for academic success.
- Professor Sophie Scott – her pre-lunch keynote discussed how laughter acts as an emotional regulator in relationships. As Victor Borge put it “laughter is the shortest distance between two people.”
- Kevin Squibb – gave a quick introduction to Hattie’s idea of Visible Learning, leading to discussion of how to take create assessment capable learners.
- Drew Thomson – examined the importance of relationships between colleagues and looked at how staff can maximise the impact of those around them.
- Bukky Yusuf – spoke about key strategies to establish effective learning behaviours with disaffected pupils in her seminar titled ‘Challenging classes and the lessons I learnt.’









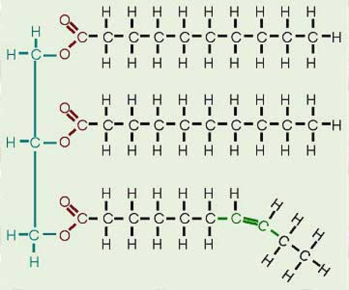
 These are characterised by a glycerol molecule with three fatty acid ‘tails’. The bond between the glycerol and fatty acid is known as an ester bond and is formed by a condensation reaction where water is produced. Hydrolysis is the reverse process and used to digest triglycerides. This is very similar to condensation and hydrolysis of
These are characterised by a glycerol molecule with three fatty acid ‘tails’. The bond between the glycerol and fatty acid is known as an ester bond and is formed by a condensation reaction where water is produced. Hydrolysis is the reverse process and used to digest triglycerides. This is very similar to condensation and hydrolysis of