Carbohydrates are excellent examples of monomers and polymers. Their one unit monomers are known as monosaccharides. Some examples of monosaccharides include:
- α-glucose
- β-glucose
- Galactose
- Fructose
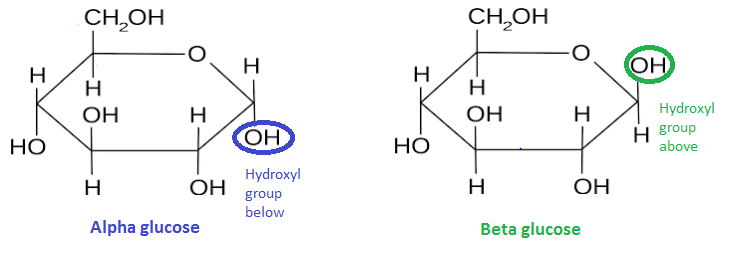
α-glucose and β-glucose are an example of a structural isomer, both have the same chemical formula, C6H12O6 but the atoms are arranged slightly differently.
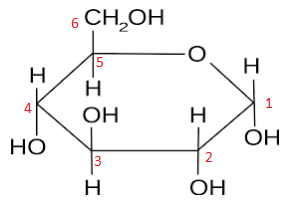
alpha glucose
The diagram to the left shows the structural arrangement of atoms in α-glucose. Although not shown on the diagram, at each corner of the hexagon between the -H and -OH (or hydroxyl group) there is a carbon atom. These have been labelled clockwise as carbon number 1, carbon number 2 and so on. The only difference between α-glucose and β-glucose is the position of the -OH on carbon number 1. In α-glucose the hydroxyl group is below the plane of the ring, e.g. the H is above it. Whereas in β-glucose, the hydroxyl group is above the plane of the ring, e.g. the H is below it. One way of remembering this is by invoking the Swedish super group ABBA. Alpha (the -OH is) Below (carbon 1), Beta (the -OH is) Above (carbon 1). Hence ABBA, alpha below, beta above. The diagram below summarise this information.
Disaccharides are made of two monosaccharides joined by a glycosidic bond. Three common disaccharides are shown below, along with the mononsaccharides that make them up.
- Maltose, made from two α-glucose molecules
- Sucrose, made from one α-glucose and one fructose molecule
- Lactose, made from one α-glucose and one galactose molecule
During the reaction between two monosacchrides that results in a disaccharide, water is formed. Therefore we call it a condensation reaction. The process of making maltose is outlined in the diagram below:
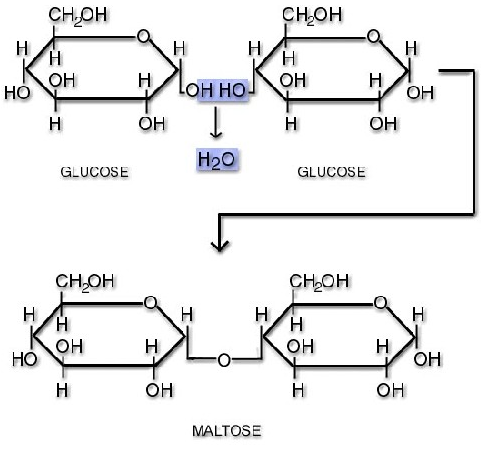
The highlighted -H and -OH groups form the water and leave a -O- that joins the two monosaccharides, which is the glycosidic bond. The glycosidic bond’s precise position is between carbon number 1 on the left hand moecule and carbon number 4 on the right hand molecule, making a 1-4 glycosidic bond. The opposite of this process, e.g. splitting a disaccharide into two monosaccharides with the addition of water, is called hydrolysis.
In terms of a balanced chemical equation for condensation we show the following, note that the disaccharide has two fewer hydrogens and one fewer oxygen, which have formed the water.
C6H12O6 + C6H12O6 → C12H22O11 + H2O
Polysaccharides are carbohydrates with three or more monosaccharides joined together with glycosidic bonds. They are formed by condensation reactions and can be broken down by hydrolysis. Three common polysaccharides are:
Glycogen, a storage molecule found in animals made from α-glucose. Its highly branched and compact shape make it very well suited to this function. The diagram to the right represents this structure, with each circle representing α-glucose.
Starch, a storage molecule found in animals made from α-glucose. It is made up of linear chains of amylose and branched amylopectin chains. Starch is insoluble, meaning that it does not effect the water potential of the cells it is stored in, so does not effect osmosis.
Cellulose, a structural carbohydrate that makes up plant cells’ cell wall made from β-glucose. It forms straight chains that come together as microfibrils to support the cell.
If you would like to know more about how these polysaccharides form and where the glycosidic bonds are located see appendix (i).
We can test for the presence of reducing sugars (e.g. glucose), non-reducing sugars (e.g. sucrose) and starch with the following tests:
- Test for reducing sugars – add sample to blue Benedict’s solution and heat above 80ºC / boil. If reducing sugars are present, a red precipitate forms. Small amounts result in a green colour, going through yellow to orange to brick red.
- Test for non-reducing sugars – first carry out the test for reducing sugars, but you will get a negative result (the Benedict’s solution remains blue). Then add dilute hyrdochloric acid to some of the original sample . Repeat the test for reducing sugars and you should get a positive result, e.g. it turn red.
- Test for starch – add iodine solution to your sample. It should change colour from orange/brown to black/blue.
Glossary
- Cellulose: polysaccharide that forms the cell wall in plants, made of β-glucose.
- Condensation reaction: a chemical reaction involving the joining together of two molecules by removal of a water molecule.
- Disaccharide: a sugar molecule consisting of two monosaccharides joined together by a glycosidic bond.
- Glucose: a monosachharide that has two isomers, α-glucose and β-glucose.
- Glycogen: a polysaccharide made of many glucose molecules linked together, that acts as a glucose store in liver and muscle cells.
- Glycosidic bond: a C-O-C link between two monosaccharide molecules, formed by a condenstaion reaction.
- Hyrdolysis: a reaction in which a complex molecule is broken down to simpler ones, involving the addition of water.
- Hydroxyl group: a pair of atoms (O and H) found in carbohydrates and other molecules.
- Monosaccharide: a molecule consisting of a single sugar unit with the general formula (CH2O)n.
- Polysaccharide: a polymer whose subunits are monosaccharides joined together by glycosidic bonds.
- Starch: polysaccharide found in most green plants as an energy store, formed from chains of amylose and amylopectin.
Appendix (i)
The formation of polysaccharides.
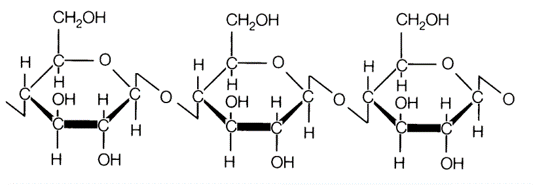
Cellulose forms 1-4 glycosidic bonds to make an unbranched chain, see below:
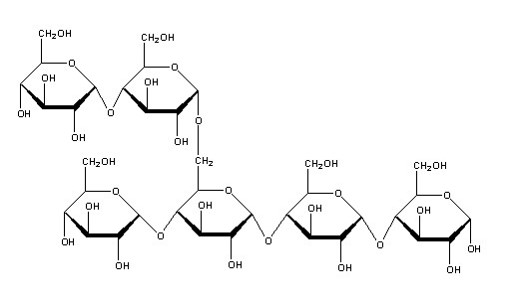
Glycogen forms a mixture of 1-4 and 1-6 glycosidic bonds, hence the branching:
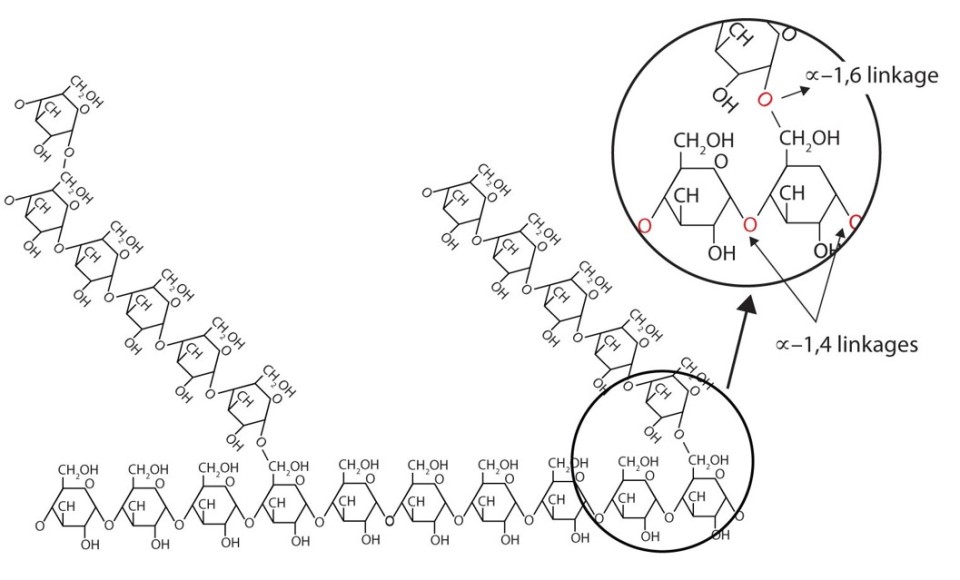
The amylose in starch forms 1-4 glycosidic bonds, so is unbranched. However, the amylopectin branches are caused by 1-6 glycosidic bonds: